Isoniazid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Hydra, Hyzyd, Isovit, others |
| Other names | isonicotinic acid hydrazide, isonicotinyl hydrazine, INH, INAH, INHA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682401 |
| License data | |
| Pregnancy category |
|
intravenous | |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Very low (0–10%) |
| Metabolism | liver; CYP450: 2C19, 3A4 inhibitor |
| Elimination half-life | 0.5–1.6h (fast acetylators), 2-5h (slow acetylators) |
| Excretion | urine (primarily), feces |
| Identifiers | |
| |
JSmol) | |
| |
| |
| (verify) | |
Isoniazid, also known as isonicotinic acid hydrazide (INH), is an
History
First synthesis was described in 1912.[6] A. Kachugin invented the drug against tuberculosis under name Tubazid in 1949. Three pharmaceutical companies unsuccessfully attempted to patent the drug at the same time,[7] the most prominent one being Roche, which launched its version, Rimifon, in 1952.[8]
The drug was first tested at Many Farms, a Navajo community in Arizona, due to the Navajo reservation's tuberculosis problem and because the population had not previously been treated with streptomycin, the main tuberculosis treatment at the time.[9] The research was led by Walsh McDermott, an infectious disease researcher with an interest in public health, who had previously taken isoniazid to treat his own tuberculosis.[10]
Isoniazid and a related drug, iproniazid, were among the first drugs to be referred to as antidepressants.[11]
It is on the
Medical uses
Tuberculosis
Isoniazid is often used to treat latent and active tuberculosis infections. In persons with isoniazid-sensitive Mycobacterium tuberculosis infection, drug regimens based on isoniazid are usually effective when persons adhere to the prescribed treatment. However, in persons with isoniazid-resistant Mycobacterium tuberculosis infection, drug regimens based on isoniazid have a high rate of failure.[14]
Isoniazid has been approved as prophylactic therapy for the following populations:
- People with HIV infection and a PPD (purified protein derivative) reaction of at least 5 mm induration
- Contacts of people with tuberculosis and who have a PPD reaction at least 5 mm induration
- People whose PPD reactions convert from negative to positive in a two-year period – at least 10 mm induration for those up to 35 years of age, and at least 15 mm induration for those at least 35 years old
- People with pulmonary damage on their chest X-ray that is likely to be due to healed tuberculosis and also have a PPD reaction at least 5 mm induration
- Injection drug users whose HIV status is negative who have a PPD reaction at least 10 mm induration
- People with a PPD of greater than or equal to 10 mm induration who are foreign-born from high prevalence geographical regions, low-income populations, and patients residing in long-term facilities[15][16]
Isoniazid can be used alone or in combination with
Non-tuberculous mycobacteria
Isoniazid was widely used in the treatment of Mycobacterium avium complex as part of a regimen including rifampicin and ethambutol.[18] Evidence suggests that isoniazid prevents mycolic acid synthesis in M. avium complex as in M. tuberculosis[19] and although this is not bactericidal to M. avium complex, it greatly potentiates the effect of rifampicin. The introduction of macrolides led to this use greatly decreasing. However, since rifampicin is broadly underdosed in M. avium complex treatment, this effect may be worth re-investigating.[20]
Special populations
It is recommended that women with active tuberculosis who are pregnant or breastfeeding take isoniazid. Preventive therapy should be delayed until after giving birth.[21] Nursing mothers excrete a relatively low and non-toxic concentration of INH in breast milk, and their babies are at low risk for side effects. Both pregnant women and infants being breastfed by mothers taking INH should take vitamin B6 in its pyridoxine form to minimize the risk of peripheral nerve damage.[22] Vitamin B6 is used to prevent isoniazid-induced B6 deficiency and neuropathy in people with a risk factor, such as pregnancy, lactation, HIV infection, alcoholism, diabetes, kidney failure, or malnutrition.[23]
People with liver dysfunction are at a higher risk for hepatitis caused by INH, and may need a lower dose.[21]
Levels of liver enzymes in the bloodstream should be frequently checked in daily alcohol drinkers, pregnant women, IV drug users, people over 35, and those who have chronic liver disease, severe kidney dysfunction, peripheral neuropathy, or HIV infection since they are more likely to develop hepatitis from INH.[21][24]
Side effects
Up to 20% of people taking isoniazid experience
Asymptomatic elevation of serum liver enzyme concentrations occurs in 10% to 20% of people taking INH, and liver enzyme concentrations usually return to normal even when treatment is continued.[26] Isoniazid has a boxed warning for severe and sometimes fatal hepatitis, which is age-dependent at a rate of 0.3% in people 21 to 35 years old and over 2% in those over age 50.[15][27] Symptoms suggestive of liver toxicity include nausea, vomiting, abdominal pain, dark urine, right upper quadrant pain, and loss of appetite.[15] Black and Hispanic women are at higher risk for isoniazid-induced hepatotoxicity.[15] When it happens, isoniazid-induced liver toxicity has been shown to occur in 50% of patients within the first 2 months of therapy.[28]
Some recommend that liver function should be monitored carefully in all people receiving it,[21] but others recommend monitoring only in certain populations.[26][29][30]
Headache, poor concentration, weight gain, poor memory, insomnia, and depression have all been associated with isoniazid use.[31] All patients and healthcare workers should be aware of these serious side effects, especially if suicidal ideation or behavior are suspected.[31][32][33]
Isoniazid is associated with pyridoxine (vitamin B6) deficiency because of its similar structure. Isoniazid is also associated with increased excretion of pyridoxine. Pyridoxal phosphate (a derivative of pyridoxine) is required for δ-aminolevulinic acid synthase, the enzyme responsible for the rate-limiting step in heme synthesis. Therefore, isoniazid-induced pyridoxine deficiency causes insufficient heme formation in early red blood cells, leading to sideroblastic anemia.[23]
Isoniazid was found to significantly elevate the in vivo concentration of
Drug interactions
People taking isoniazid and acetaminophen are at risk of acetaminophen toxicity. Isoniazid is thought to induce a liver enzyme which causes a larger amount of acetaminophen to be metabolized to a toxic form.[35][36]
Isoniazid decreases the metabolism of carbamazepine, thus slowing down its clearance from the body. People taking carbamazepine should have their carbamazepine levels monitored and, if necessary, have their dose adjusted accordingly.[37]
It is possible that isoniazid may decrease the serum levels of ketoconazole after long-term treatment. This is seen with the simultaneous use of rifampin, isoniazid, and ketoconazole.[38]
Isoniazid may increase the amount of phenytoin in the body. The doses of phenytoin may need to be adjusted when given with isoniazid.[39][40]
Isoniazid may increase the plasma levels of theophylline. There are some cases of theophylline slowing down isoniazid elimination. Both theophylline and isoniazid levels should be monitored.[41]
Valproate levels may increase when taken with isoniazid. Valproate levels should be monitored and its dose adjusted if necessary.[39]
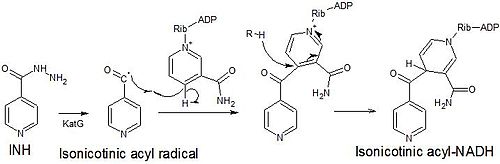
Mechanism of action
Isoniazid is a
Isoniazid is
Isoniazid is a mild non-selective monoamine oxidase inhibitor(MAO-I).[47]
Metabolism
Isoniazid reaches therapeutic concentrations in serum,
Preparation
Isoniazid is an
It can in theory be made from methyl isonicotinate, which is labelled a semiochemical.
Brand names
Hydra, Hyzyd, Isovit, Laniazid, Nydrazid, Rimifon, and Stanozide.[50]
Other uses
Chromatography
Isonicotinic acid hydrazide is also used in chromatography to differentiate between various degrees of conjugation in organic compounds barring the ketone functional group.[51] The test works by forming a hydrazone which can be detected by its bathochromic shift.[citation needed]
Dogs
Isoniazid may be used for dogs, but there have been concerns it can cause seizures.[52]
References
- ^ "Isoniazid (Nydrazid) Use During Pregnancy". Drugs.com. 7 October 2019. Retrieved 24 January 2020.
- FDA. Retrieved 22 Oct 2023.
- ^ "Drug and medical device highlights 2018: Helping you maintain and improve your health". Health Canada. 14 October 2020. Retrieved 17 April 2024.
- ^ a b c d e "Isoniazid". The American Society of Health-System Pharmacists. Archived from the original on 20 December 2016. Retrieved 8 December 2016.
- ISBN 9789241547659.
- PMID 32166015.
- S2CID 43789954.
- ^ "History". rocheusa.com. Roche USA. Archived from the original on 2007-12-12.
- S2CID 30166423.
- ISBN 978-0-309-04198-0.
- PMID 18321627.
- hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ISBN 9789241515528.
- PMID 27865891.
- ^ a b c d e f g "Isoniazid (package insert)". 30 March 2023.
- ^ "The Use of Preventive Therapy for Tuberculosis Infection in the United States – Recommendations of the Advisory Committee for Elimination of Tuberculosis". Morbidity and Mortality Weekly Report. 39 (RR-8): 9–12. May 18, 1990. Archived from the original on 2 March 2016. Retrieved 22 February 2016.
- ^ )
- PMID 11182006.
- S2CID 13764717.
- PMID 22744719.
- ^ a b c d "Isoniazid tablet". DailyMed. 18 October 2018. Archived from the original on 13 March 2019. Retrieved 24 January 2020.
- S2CID 10479433.
- ^ PMID 16788441.
- S2CID 36384722.
- ISBN 9781609137137.
- ^ a b "Latent Tuberculosis Infection: A Guide for Primary Health Care Providers". cdc.gov. Center for Disease Control. Archived from the original on 25 March 2016. Retrieved 25 March 2016.
- ^ Trevor, A. & Katzung, B. (2013). Katzung & Trevor's Pharmacology: examination & board review (10th ed., p. 417). New York. McGraw-Hill Medical, Lange.
- ^ "Isoniazid UpToDate". Archived from the original on 2015-10-25.
- ^ "Treatment of Tuberculosis – Guidelines (4th ed.)" (PDF). who.int. World Health Organization. Archived (PDF) from the original on 4 April 2016. Retrieved 25 March 2016.
- PMID 9797751.
- ^ S2CID 73122253.
- S2CID 31383347.
- S2CID 28637999.
- S2CID 215809029.
- PMID 2240884.
- PMID 2218067.
- PMID 2036861.
- PMID 8357281.
- ^ S2CID 22218366.
- PMID 8173779.
- PMID 3817069.
- PMID 19139099.
- PMID 15273113.
- PMID 19039139.
- PMID 19686043.
- ISBN 9780781741187.
- PMID 8056994.
- ISBN 9780815515265.
- PMID 13345683.
- ^ "Drugs@FDA". fda.gov. United States Food and Drug Administration. Archived from the original on 14 August 2012. Retrieved 22 August 2016.
- PMID 32166015.
- ISBN 978-0323241946– via Google Books.
Further reading
- Romero JA, Kuczler FJ (February 1998). "Isoniazid overdose: recognition and management". American Family Physician. 57 (4): 749–752. PMID 9490997. Archived from the originalon 2011-11-01. Retrieved 2005-12-13.
External links
 Media related to Isoniazid at Wikimedia Commons
Media related to Isoniazid at Wikimedia Commons