Actinium
 | ||||||||||||||||||||||||||||||||||
| Actinium | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ækˈtɪniəm/ | |||||||||||||||||||||||||||||||||
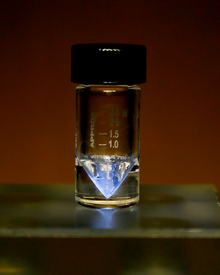
| Appearance | silvery-white, glowing with an eerie blue light;[1] sometimes with a golden cast[2] | |||||||||||||||||||||||||||||||||
| Mass number | [227] | |||||||||||||||||||||||||||||||||
| Actinium in the periodic table | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
Discovery and first isolation | Friedrich Oskar Giesel (1902, 1903) | |||||||||||||||||||||||||||||||||
| Named by | André-Louis Debierne (1899) | |||||||||||||||||||||||||||||||||
| Isotopes of actinium | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
Actinium is a
A soft, silvery-white
History
André-Louis Debierne, a French chemist, announced the discovery of a new element in 1899. He separated it from pitchblende residues left by Marie and Pierre Curie after they had extracted radium. In 1899, Debierne described the substance as similar to titanium[5] and (in 1900) as similar to thorium.[6] Friedrich Oskar Giesel found in 1902[7] a substance similar to lanthanum and called it "emanium" in 1904.[8] After a comparison of the substances' half-lives determined by Debierne,[9] Harriet Brooks in 1904, and Otto Hahn and Otto Sackur in 1905, Debierne's chosen name for the new element was retained because it had seniority, despite the contradicting chemical properties he claimed for the element at different times.[10][11]
Articles published in the 1970s
The name actinium originates from the
Properties
Actinium is a soft, silvery-white,
The first element of the actinides, actinium gave the set its name, much as lanthanum had done for the lanthanides. The actinides are much more diverse than the lanthanides[22] and therefore it was not until 1945 that the most significant change to Dmitri Mendeleev's periodic table since the recognition of the lanthanides, the introduction of the actinides, was generally accepted after Glenn T. Seaborg's research on the transuranium elements[23] (although it had been proposed as early as 1892 by British chemist Henry Bassett).[24]
Actinium reacts rapidly with oxygen and moisture in air forming a white coating of
Chemical compounds
Due to actinium's intense radioactivity, only a limited number of actinium compounds are known. These include:
| Formula | color | symmetry | space group | No | Pearson symbol | a (pm) | b (pm) | c (pm) | Z | density, g/cm3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Ac | silvery | fcc[31] | Fm3m | 225 | cF4 | 531.1 | 531.1 | 531.1 | 4 | 10.07 |
| AcH2 | unknown | cubic[31] | Fm3m | 225 | cF12 | 567 | 567 | 567 | 4 | 8.35 |
| Ac2O3 | white[17] | trigonal[32]
|
P3m1 | 164 | hP5 | 408 | 408 | 630 | 1 | 9.18 |
| Ac2S3 | black | cubic[33] | I43d | 220 | cI28 | 778.56 | 778.56 | 778.56 | 4 | 6.71 |
| AcF3 | white[34] | P3c1 | 165 | hP24 | 741 | 741 | 755 | 6 | 7.88 | |
| AcCl3 | white | hexagonal[30][35] | P63/m | 165 | hP8 | 764 | 764 | 456 | 2 | 4.8 |
| AcBr3 | white[30] | hexagonal[35] | P63/m | 165 | hP8 | 764 | 764 | 456 | 2 | 5.85 |
| AcOF | white[36] | cubic[30] | Fm3m | 593.1 | 8.28 | |||||
| AcOCl | white | tetragonal[30] | 424 | 424 | 707 | 7.23 | ||||
| AcOBr | white | tetragonal[30] | 427 | 427 | 740 | 7.89 | ||||
| AcPO4·0.5H2O | unknown | hexagonal[30] | 721 | 721 | 664 | 5.48 |
Here a, b and c are lattice constants, No is space group number and Z is the number of formula units per unit cell. Density was not measured directly but calculated from the lattice parameters.
Oxides
Halides
- AcF3 + 2 NH3 + H2O → AcOF + 2 NH4F
Actinium trichloride is obtained by reacting actinium hydroxide or
Reaction of aluminium bromide and actinium oxide yields actinium tribromide:
- Ac2O3 + 2 AlBr3 → 2 AcBr3 + Al2O3
and treating it with ammonium hydroxide at 500 °C results in the oxybromide AcOBr.[30]
Other compounds
Actinium hydride was obtained by reduction of actinium trichloride with potassium at 300 °C, and its structure was deduced by analogy with the corresponding LaH2 hydride. The source of hydrogen in the reaction was uncertain.[37]
Mixing
Isotopes
Naturally occurring actinium is principally composed of two radioactive
Purified 227
Ac comes into equilibrium with its decay products after about a half of year. It decays according to its 21.772-year half-life emitting mostly beta (98.62%) and some alpha particles (1.38%);
| Isotope | Production | Decay | Half-life |
|---|---|---|---|
| 221Ac | 232Th(d,9n)→225Pa(α)→221Ac | α | 52 ms |
| 222Ac | 232Th(d,8n)→226Pa(α)→222Ac | α | 5.0 s |
| 223Ac | 232Th(d,7n)→227Pa(α)→223Ac | α | 2.1 min |
| 224Ac | 232Th(d,6n)→228Pa(α)→224Ac | α | 2.78 hours |
| 225Ac | 232Th(n,γ)→233Th(β−)→233Pa(β−)→233U(α)→229Th(α)→225Ra(β−)→225Ac | α | 10 days |
| 226Ac | 226Ra(d,2n)→226Ac | α, β− electron capture |
29.37 hours |
| 227Ac | 235U(α)→231Th(β−)→231Pa(α)→227Ac | α, β− | 21.77 years |
| 228Ac | 232Th(α)→228Ra(β−)→228Ac | β− | 6.15 hours |
| 229Ac | 228Ra(n,γ)→229Ra(β−)→229Ac | β− | 62.7 min |
| 230Ac | 232Th(d,α)→230Ac | β− | 122 s |
| 231Ac | 232Th(γ,p)→231Ac | β− | 7.5 min |
| 232Ac | 232Th(n,p)→232Ac | β− | 119 s |
Occurrence and synthesis

Actinium is found only in traces in
The low natural concentration, and the close similarity of physical and chemical properties to those of lanthanum and other lanthanides, which are always abundant in actinium-bearing ores, render separation of actinium from the ore impractical. The most concentrated actinium sample prepared from raw material consisted of 7 micrograms of 227Ac in less than 0.1 milligrams of La2O3, and complete separation was never achieved.[41] Instead, actinium is prepared, in milligram amounts, by the neutron irradiation of 226Ra in a nuclear reactor.[40][42]
The reaction yield is about 2% of the radium weight. 227Ac can further capture neutrons resulting in small amounts of 228Ac. After the synthesis, actinium is separated from radium and from the products of decay and nuclear fusion, such as thorium, polonium, lead and bismuth. The extraction can be performed with
225Ac was first produced artificially at the Institute for Transuranium Elements (ITU) in Germany using a cyclotron and at St George Hospital in Sydney using a linac in 2000.[44] This rare isotope has potential applications in radiation therapy and is most efficiently produced by bombarding a radium-226 target with 20–30 MeV deuterium ions. This reaction also yields 226Ac which however decays with a half-life of 29 hours and thus does not contaminate 225Ac.[45]
Actinium metal has been prepared by the reduction of actinium fluoride with lithium vapor in vacuum at a temperature between 1100 and 1300 °C. Higher temperatures resulted in evaporation of the product and lower ones lead to an incomplete transformation. Lithium was chosen among other alkali metals because its fluoride is most volatile.[14][17]
Applications
Owing to its scarcity, high price and radioactivity, 227Ac currently has no significant industrial use, but 225Ac is currently being studied for use in cancer treatments such as targeted alpha therapies.[14][28] 227Ac is highly radioactive and was therefore studied for use as an active element of radioisotope thermoelectric generators, for example in spacecraft. The oxide of 227Ac pressed with beryllium is also an efficient neutron source with the activity exceeding that of the standard americium-beryllium and radium-beryllium pairs.[46] In all those applications, 227Ac (a beta source) is merely a progenitor which generates alpha-emitting isotopes upon its decay. Beryllium captures alpha particles and emits neutrons owing to its large cross-section for the (α,n) nuclear reaction:
The 227AcBe neutron sources can be applied in a
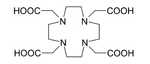
225Ac is applied in medicine to produce
The medium half-life of 227Ac (21.77 years) makes it a very convenient radioactive isotope in modeling the slow vertical mixing of oceanic waters. The associated processes cannot be studied with the required accuracy by direct measurements of current velocities (of the order 50 meters per year). However, evaluation of the concentration depth-profiles for different isotopes allows estimating the mixing rates. The physics behind this method is as follows: oceanic waters contain homogeneously dispersed 235U. Its decay product, 231Pa, gradually precipitates to the bottom, so that its concentration first increases with depth and then stays nearly constant. 231Pa decays to 227Ac; however, the concentration of the latter isotope does not follow the 231Pa depth profile, but instead increases toward the sea bottom. This occurs because of the mixing processes which raise some additional 227Ac from the sea bottom. Thus analysis of both 231Pa and 227Ac depth profiles allows researchers to model the mixing behavior.[54][55]
There are theoretical predictions that AcHx hydrides (in this case with very high pressure) are a candidate for a near room-temperature superconductor as they have Tc significantly higher than H3S, possibly near 250 K.[56]
Precautions
227Ac is highly radioactive and experiments with it are carried out in a specially designed laboratory equipped with a tight
See also
Notes
References
- ^ Wall, Greg (8 September 2003). "C&EN: It's Elemental: The Periodic Table - Actinium". C&EN: It's Elemental: The Periodic Table. Chemical and Engineering News. Retrieved 2 June 2011.
- ^ ISBN 978-1-4020-3555-5.
- ISBN 978-1-62708-155-9.
- .
- ^ Debierne, André-Louis (1899). "Sur un nouvelle matière radio-active". Comptes Rendus (in French). 129: 593–595.
- ^ Debierne, André-Louis (1900–1901). "Sur un nouvelle matière radio-actif – l'actinium". Comptes Rendus (in French). 130: 906–908.
- .
- .
- ^ Debierne, André-Louis (1904). "Sur l'actinium". Comptes Rendus (in French). 139: 538–540.
- .
- .
- ^ S2CID 144651011.
- ^ S2CID 94016074.
- ^ ISBN 0-8493-0486-5.
- ISBN 978-0-549-79554-4.
- ISBN 978-0-470-48788-4.
- ^ .
- ^ a b Actinium, in Encyclopædia Britannica, 15th edition, 1995, p. 70
- ISBN 0-12-607716-9pp. 289–291
- ISBN 978-0-691-13504-5.
- .
- .
- PMID 17842184.
- )
- ^ a b c Actinium, Great Soviet Encyclopedia (in Russian)
- S2CID 249433367.
- .
- ^ PMID 27768109.
- PMID 28386595.
- ^ .
- ^ OSTI 4397640.
- ^ .
- (PDF) from the original on 9 October 2022.
- ^ Meyer, p. 71
- ^ .
- ^ a b Meyer, pp. 87–88
- ^ Meyer, p. 43
- ^ .
- ^ .
- ^ ISBN 978-0-08-037941-8.
- ISBN 978-94-007-0210-3.
- ISBN 978-0-12-023631-2.
- ^ PMID 15763472.
- PMID 19135381.
- ISBN 0-89603-978-1, p. 336
- ISBN 0-471-64952-X, pp. 470–471
- ISBN 81-203-1729-7p. 108
- ISBN 81-7211-200-9pp. 202 ff
- doi:10.1139/p57-075.
- PMID 10425108.
- S2CID 11782419.
- (PDF) from the original on 9 October 2022.
- PMID 15240541.
- S2CID 4344946.
- .
- S2CID 4620593.
- doi:10.2172/4406766.
- ISBN 978-3527306732.
Bibliography
- Meyer, Gerd and Morss, Lester R. (1991) Synthesis of lanthanide and actinide compounds, Springer. ISBN 0-7923-1018-7
External links
- Actinium at The Periodic Table of Videos(University of Nottingham)
- NLM Hazardous Substances Databank – Actinium, Radioactive
- Actinium in Kirby, H. W.; Morss, L. R. (2006). Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer. ISBN 978-1-4020-3555-5.

![{\displaystyle {\ce {^{226}_{88}Ra + ^{1}_{0}n -> ^{227}_{88}Ra ->[\beta^-][42.2 \ {\ce {min}}] ^{227}_{89}Ac}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0971e4ce21fbf7bb4673856bff635b1a64d11fb2)
