Organotin chemistry

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are
Structure
Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful.
Organic derivatives of tin(IV)
The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R''' have been resolved into individual enantiomers.[3]
Organotin halides
Organotin chlorides have the formula R4−nSnCln for values of n up to 3. Bromides, iodides, and fluorides are also known, but are less important. These compounds are known for many R groups. They are always tetrahedral. The tri- and dihalides form adducts with good Lewis bases such as pyridine. The fluorides tend to associate such that dimethyltin difluoride forms sheet-like polymers. Di- and especially tri-organotin halides, e.g. tributyltin chloride, exhibit toxicities approaching that of hydrogen cyanide.[4]
Organotin hydrides
Organotin hydrides have the formula R4−nSnHn for values of n up to 3. The parent member of this series, stannane (SnH4), is an unstable colourless gas. Stability is correlated with the number of organic substituents. Tributyltin hydride is used as a source of hydride radical in some organic reactions.
Organotin oxides and hydroxides
Organotin oxides and hydroxides are common products from the hydrolysis of organotin halides. Unlike the corresponding derivatives of silicon and germanium, tin oxides and hydroxides often adopt structures with penta- and even hexacoordinated tin centres, especially for the diorgano- and monoorgano derivatives. The group SnIV−O−SnIV is called a
- 2 R3SnOH ⇌ R3SnOSnR3 + H2O
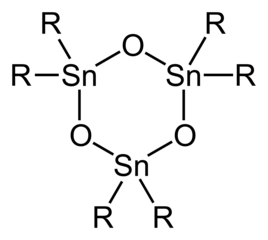
With only two organic substituents on each Sn centre, the diorganotin oxides and hydroxides are structurally more complex than the triorgano derivatives.
-
Idealized structure of trimeric diorganotin oxide.
-
Ball-and-stick model for (((CH3)3C)2SnO)3.
-
Structure of diorganotin oxide, highlighting the extensive intermolecular bonding.
Hypercoordinated stannanes
Unlike carbon(IV) analogues but somewhat like silicon compounds, tin(IV) can also be
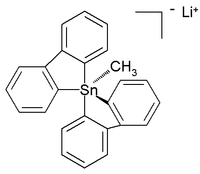
The all-organic penta- and hexaorganostannates(IV) have even been characterized,[7] while in the subsequent year a six-coordinated tetraorganotin compound was reported.[8] A crystal structure of room-temperature stable (in argon) all-carbon pentaorganostannate(IV) was reported as the lithium salt with this structure:[9]
In this distorted
Triorganotin cations
Some reactions of triorganotin halides implicate a role for R3Sn+ intermediates. Such cations are analogous to carbocations. They have been characterized crystallographically when the organic substituents are large, such as 2,4,6-triisopropylphenyl.[10]
Tin radicals (organic derivatives of tin(III))
Tin radicals, with the formula R3Sn, are called stannyl radicals.[2] They are invoked as intermediates in certain atom-transfer reactions. For example, tributyltin hydride (tris(n-butyl)stannane) serves as a useful source of "hydrogen atoms" because of the stability of the tributytin radical.[11]
Organic derivatives of tin(II)
Organotin(II) compounds are somewhat rare. Compounds with the empirical formula SnR2 are somewhat fragile and exist as rings or polymers when R is not bulky. The polymers, called polystannanes, have the formula (SnR2)n.
In principle, compounds of tin(II) might be expected to form a tin analogues of
- 2 R2Sn ⇌ R2Sn=SnR2
Stannenes, compounds with tin-carbon double bonds, are exemplified by derivatives of stannabenzene. Stannoles, structural analogs of cyclopentadiene, exhibit little C-Sn double bond character.
Organic derivatives of tin(I)
Compounds of Sn(I) are rare and only observed with very bulky ligands. One prominent family of cages is accessed by pyrolysis of the 2,6-diethylphenyl-substituted tristannylene [Sn(C6H3-2,6-Et2)2]3, which affords the cubane-type cluster and a prismane. These cages contain Sn(I) and have the formula [Sn(C6H3-2,6-Et2)]n where n = 8, 10 and Et stands for ethyl group.[13] A stannyne contains a tin atom to carbon group atom triple bond (e.g. R−Sn≡C−R and R−Sn≡Si−R), and a distannyne a triple bond between two tin atoms (R−Sn≡Sn−R). Distannynes only exist for extremely bulky substituents. Unlike alkynes, the C−Sn≡Sn−C core of these distannynes are nonlinear, although they are planar. The Sn-Sn distance is 3.066(1) Å, and the Sn-Sn-C angles are 99.25(14)°. Such compounds are prepared by reduction of bulky aryltin(II) halides.[14]

Grey balls: C
Magenta (largest) balls: Sn
Structure of an Ar10Sn10 "prismane", a compound containing Sn(I) (Ar = 2,6-diethylphenyl).
Preparation
Organotin compounds can be synthesised by numerous methods.
- 4 CH3CH2MgBr + SnCl4 → (CH3CH2)4Sn + 4 MgClBr
The symmetrical tetraorganotin compounds, especially tetraalkyl derivatives, can then be converted to various mixed chlorides by
- 3 R4Sn + SnCl4 → 4 R3SnCl
- R4Sn + SnCl4 → 2 R2SnCl2
- R4Sn + 3 SnCl4 → 4 RSnCl3
A related method involves redistribution of tin halides with
The mixed organo-halo tin compounds can be converted to the mixed organic derivatives, as illustrated by the synthesis of dibutyldivinyltin:[17]
- Bu2SnCl2 + 2 CH2=CHMgBr → Bu2Sn(CH=CH2)2 + 2 MgBrCl
The organotin hydrides are generated by reduction of the mixed alkyl chlorides. For example, treatment of dibutyltin dichloride with lithium aluminium hydride gives the dibutyltin dihydride, a colourless distillable oil:[18]
- 2 Bu2SnCl2 + Li[AlH4] → 2 Bu2SnH2 + Li[AlCl4]
The
Hydrostannylation involves the metal-catalyzed addition of tin hydrides across unsaturated substrates.[19]
Reactions
Important reactions, discussed above, usually focus on organotin
In "pure"
:- R1−X + R2−Sn(R3)3 R1−R2 + X−Sn(R3)3
Organotin compounds are also used extensively in radical chemistry (e.g. radical cyclizations, Barton–McCombie deoxygenation, Barton decarboxylation, etc.).
Applications
An organotin compound is commercially applied as stabilizers in
Biological applications
"
Tributyltin compounds were once widely used as marine anti-
- Organotin compounds
-
Tetrabutyltin colorless oil, precursor to the other butyl-tin compounds
-
Tributyltin oxide, a colorless to pale yellow liquid used in wood preservation
-
Triphenyltin acetate, an off-white crystalline solid, used as an insecticide and a fungicide
-
Triphenyltin chloride, a highly toxic white solid, used as a biocide
-
Trimethyltin chloride, a toxic white solid, once used as a biocide
-
Triphenyltin hydroxide, an off-white powder, used as a fungicide
-
miticide
-
Hexamethylditin used as an intermediate in chemical synthesis
-
Tetraethyltin, boiling point 63–65° /12 mm[clarification needed] is a catalyst.[24] The "Et" symbol stands for ethyl group.
Toxicity
The toxicities of tributyltin and triphenyltin derivative compounds are comparable to that of
In contrast to the triorganotin compounds, monoorgano, diorgano- and tetraorganotin compounds are far less dangerous,[4] although DBT may be immunotoxic.[25]
See also
References
- .
- ^ ISBN 978-3-527-31023-4
- .
- ^ ISBN 978-3527306732.
- ^ "Stannanol | H4OSn | ChemSpider".
- ^ .
- .
- .
- PMID 17705378.
- ISBN 978-0-08-037941-8.
- ISBN 0-12-352651-5
- .
- .
- ^ Sander H.L. Thoonen; Berth-Jan Deelman; Gerard van Koten (2004). "Synthetic aspects of tetraorganotins and organotin(IV) halides" (PDF). Journal of Organometallic Chemistry (689): 2145–2157.
- .
- .
- ISBN 0122349504.
- PMID 11749320.
- ^ Eisch 1981, pp. 156, 169.
- ISBN 978-3-527-29390-2.
- ISBN 9781847551771.
- PMID 32102175.
- ^ Organic Syntheses, Coll. Vol. 4, p.881 (1963); Vol. 36, p.86 (1956). Link
- PMID 18958157.












![Tetraethyltin, boiling point 63–65° /12 mm[clarification needed] is a catalyst.[24] The "Et" symbol stands for ethyl group.](http://upload.wikimedia.org/wikipedia/commons/thumb/1/1e/Tetraethyltin.svg/120px-Tetraethyltin.svg.png)